Nothing to See Here, Move Along
The Supreme Court’s recent unanimous decision on FDA’s tobacco-authorization authority is utterly unremarkable.
A New Era of Cosmetics Safety Regulation
Scholars consider how the Modernization of Cosmetics Regulation Act strengthens federal oversight.
Restoring FDA’s Premarket Review Powers
Scholar proposes reforms to address deficiencies in FDA’s premarket review powers.
Multi-Level Marketing, Multi-Level Accountability
Scholar urges lawmakers to hold multi-level marketing companies liable for misleading claims made by salespeople.
Addressing the Medical Device Safety Crisis
Scholar calls on FDA to prioritize public health by reforming medical device regulation.
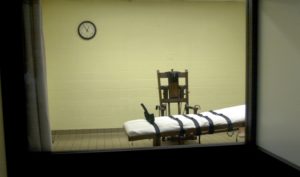
Making Drugs Safe to Kill?
Lawyer proposes a new legal framework for reviewing FDA’s refusal to regulate lethal injection drugs.
Does Cosmetics Regulation Need a Makeover?
Scholars reflect on the need for stronger regulatory oversight of cosmetics in the United States.
Smoking Out E-Cigarettes
Scholars examine the regulation of electronic cigarettes and their impact on public health.
Strengthening the Stem Cell Industry Through Better Regulation
FDA should take steps to strengthen the regenerative medicine industry.
Calls for Regulatory Approval of Edible Insects
Edible insects may become essential in the wake of the COVID-19 crisis, but FDA regulation is lacking.
Encouraging Competition Through Generic Drugs
FDA clarifies its generic drug approval process in an effort to encourage market competition.
Obligation Alleviation During the COVID-19 Crisis
The most surprising regulatory dimension of the coronavirus crisis may center on the lifting of rules.