Rationing Scarce Medical Resources
The COVID-19 pandemic raises questions about how decision-makers should distribute medical resources.
Telemedicine During the COVID-19 Crisis
With medical systems overwhelmed during the pandemic, telemedicine could be here to stay.
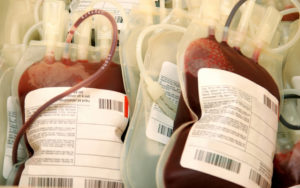
Federal Focus Shifts to COVID-19 Treatments
FDA releases guidance on using blood from recovered COVID-19 patients to treat new cases.
Your COVID-19 Immunity Papers, Please
Coronavirus immunity certificates could permit recovered persons to escape lockdown restrictions.
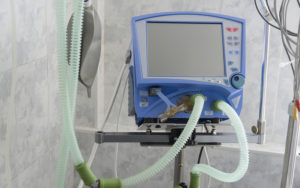
Regulating Rationing During the COVID-19 Crisis
Regulators and policymakers review old rules and propose new guidelines for rationing ventilators.
Encouraging Competition Through Generic Drugs
FDA clarifies its generic drug approval process in an effort to encourage market competition.
Combatting Covert Chemistry Operations
To curb overdoses and drug trafficking, the DEA proposes regulations for a chemical precursor to fentanyl.
Proposal to Import Prescription Drugs Faces Criticism
Critics of a proposed rule to import drugs from Canada cite safety concerns and other potential problems.
FDA Versus Our Own Stem Cells
The federal government seeks authority over stem cell treatments but risks thwarting medical advancement.
FDA Relaxes Rules on Ventilators for COVID-19
Responding to the coronavirus outbreak, a federal agency relaxes requirements on medical device manufacturers.
An Epidemic Meets a Pandemic
Amid the coronavirus, the federal government eases restrictions on prescribing controlled substances by telehealth.